CAR T-Cell Therapy for Multiple Myeloma Market to Witness Upsurge in Growth by 2034 Owing to the Launch of Emerging Therapies | DelveInsight
The dynamics of the CAR-T cell therapy market for multiple myeloma are anticipated to change due to extensive R&D activities, increased investment in research and development of new CAR-T cell therapies such as anito-cel (Arcellx and Kite), Zevor-cel (CARsgen Therapeutics), arlocabtagene autoleucel (Juno Therapeutics), P-BCMA-ALLO1 (Poseida Therapeutics/Roche), GC012F (Gracell Biopharmaceuticals), and others, and the high effectiveness of CAR-T cell therapies over other conventional drugs.
New York, USA, Oct. 08, 2025 (GLOBE NEWSWIRE) -- CAR T-Cell Therapy for Multiple Myeloma Market to Witness Upsurge in Growth by 2034 Owing to the Launch of Emerging Therapies | DelveInsight
The dynamics of the CAR-T cell therapy market for multiple myeloma are anticipated to change due to extensive R&D activities, increased investment in research and development of new CAR-T cell therapies such as anito-cel (Arcellx and Kite), Zevor-cel (CARsgen Therapeutics), arlocabtagene autoleucel (Juno Therapeutics), P-BCMA-ALLO1 (Poseida Therapeutics/Roche), GC012F (Gracell Biopharmaceuticals), and others, and the high effectiveness of CAR-T cell therapies over other conventional drugs.
DelveInsight’s CAR T-Cell Therapy for Multiple Myeloma Market Insights report includes a comprehensive understanding of current treatment practices, emerging CAR T-cell therapy for multiple myeloma, market share of individual therapies, and current and forecasted CAR T-cell therapy for multiple myeloma market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
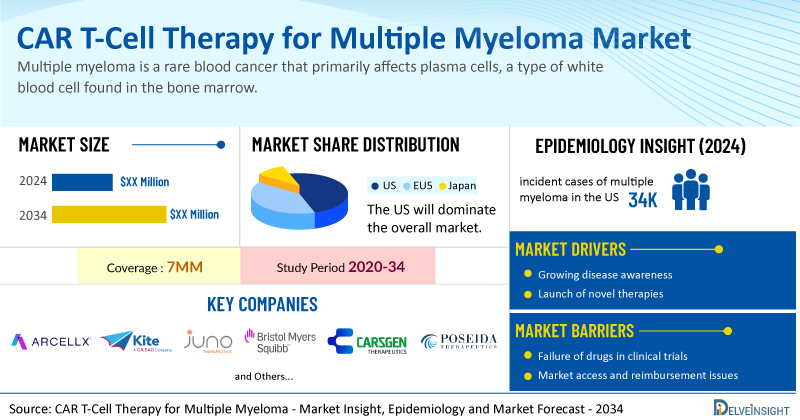
CAR T-Cell Therapy for Multiple Myeloma Market Summary
- The total CAR T-cell therapy for multiple myeloma treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of CAR T-cell therapy for multiple myeloma, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- Among the leading markets, the highest incident cases were in the US in 2024, that is nearly 34,000.
- Key CAR T-cell therapy for multiple myeloma companies, including Arcellx, Kite, Juno Therapeutics, Bristol-Myers Squibb, CARsgen Therapeutics, Poseida Therapeutics, Roche, Caribou Biosciences, Gracell Biopharmaceuticals, and others, are actively working on innovative CAR T-cell therapy for multiple myeloma.
- Some of the key CAR T-cell therapies for multiple myeloma in clinical trials include Anitocabtagene autoleucel, Arlocabtagene autoleucel, Zevor-cel (zevorcabtagene autoleucel), P-BCMA-ALLO1, CB-011, GC012F, and others. These novel CAR T-cell therapies for multiple myeloma are anticipated to enter the CAR T-cell therapy for multiple myeloma market in the forecast period and are expected to change the market.
Discover which CAR T-cell therapy for multiple myeloma medications are expected to grab the market share @ CAR T-Cell Therapy for Multiple Myeloma Market Report
Key Factors Driving the Growth of the CAR T-Cell Therapy for Multiple Myeloma Market
Rising Multiple Myeloma Incidence
The increasing number of multiple myeloma cases worldwide is a significant driver for CAR T-cell therapy adoption. In the US alone, over 32,000 new cases are diagnosed annually, highlighting the growing need for effective treatments. According to DelveInsight analysis, among the 7MM, the highest number of incident cases was reported in the US in 2024, at nearly 34,000, which are further expected to increase by 2034.
Efficacy of BCMA-Targeted Therapies
BCMA-targeted CAR T-cell therapies, such as Bristol Myers Squibb/Bluebird Bio’s ABECMA and Johnson & Johnson’s CARVYKTI, have demonstrated high response rates in treating patients with relapsed or refractory multiple myeloma. These therapies offer new hope for patients who have exhausted other treatment options.
Rising CAR T-Cell Therapy for Multiple Myeloma Clinical Trial Activity
Some of the prominent players in this field include Arcellx and Kite (a Gilead company) (anitocabtagene autoleucel, anito-cel), CARsgen Therapeutics (Zevor-cel, zevorcabtagene autoleucel), Kelonia Therapeutics (KLN-1010), Poseida Therapeutics/Roche (P-BCMA-ALLO1), Juno Therapeutics (Bristol Myers Squibb) (arlocabtagene autoleucel), Gracell Biopharmaceuticals (GC012F), Caribou Biosciences (CB-011), and others.
CAR T-Cell Therapy for Multiple Myeloma Market Analysis
CAR-T cell therapy is emerging as a highly promising treatment for multiple myeloma, particularly for patients with relapsed or refractory disease. These therapies target B-cell maturation antigen (BCMA), a protein found on the surface of multiple myeloma cells. By explicitly focusing on BCMA, CAR-T therapies have shown remarkable efficacy, achieving high response rates in patients who have already undergone and exhausted other treatment options.
Currently, two CAR-T therapies, ABECMA (idecabtagene vicleucel) and CARVYKTI (ciltacabtagene autoleucel), have received FDA approval for relapsed or refractory multiple myeloma. Notably, CARVYKTI is the first and only BCMA-targeted therapy approved by the US FDA for patients who have received at least one prior line of treatment and has become the most commercially successful CAR-T therapy for multiple myeloma, with sales in its first seven quarters surpassing historical CAR-T launches.
Despite their efficacy, CAR-T therapies are associated with safety concerns, such as cytokine release syndrome (CRS). Initial adoption may also be limited by factors including cost, convenience, and manufacturing turnaround time; however, ongoing advancements are expected to reduce side effects and accelerate production. In heavily pretreated multiple myeloma patients, CAR-T therapies are a significant focus, though bispecific antibodies have also been approved in this segment. While CAR-T therapies are often preferred, they come with their own challenges, and research continues to explore optimal sequencing with bispecific antibodies, given the high overall response rates observed for both classes.
As a novel and innovative treatment, CAR-T therapy has relatively few agents currently under clinical development for multiple myeloma. These include Anitocabtagene autoleucel (Arcellx/Kite), Zevor-cel (zevorcabtagene autoleucel) from CARsgen Therapeutics, Arlocabtagene autoleucel (Juno Therapeutics/Bristol-Myers Squibb), and others.
Learn more about the multiple myeloma treatment options @ CAR T-Cell Therapy for Multiple Myeloma Treatment Market
CAR T-Cell Therapy for Multiple Myeloma Competitive Landscape
Some of the CAR T-cell therapies for multiple myeloma in clinical trials include anitocabtagene autoleucel (anito-cel, Arcellx and Kite (a Gilead company), Zevor-cel (CT053, zevorcabtagene autoleucel) (CARsgen Therapeutics), arlocabtagene autoleucel (Juno Therapeutics [a Bristol Myers Squibb company]), P-BCMA-ALLO1 (Poseida Therapeutics/Roche), GC012F (Gracell Biopharmaceuticals), and others.
Juno Therapeutics’ Arlocabtagene autoleucel is an experimental CAR T-cell therapy that targets GPRC5D for the treatment of patients with relapsed or refractory multiple myeloma. As a GPRC5D-directed therapy, it offers a novel immunotherapeutic option for patients who have already undergone multiple prior treatments. This therapy is currently being assessed in the Phase III QUINTESSENTIAL-2 clinical trial involving adults with relapsed or refractory multiple myeloma.
Arcellx and Kite’s Anitocabtagene autoleucel is a BCMA-targeted CAR T-cell therapy that employs a unique binder known as the D-Domain. It is being investigated for patients with relapsed or refractory multiple myeloma who have received 1–3 previous lines of therapy, including both an immunomodulatory drug (IMiD) and an anti-CD38 antibody. The therapy has received Fast Track, Orphan Drug, and Regenerative Medicine Advanced Therapy designations from the US FDA. Anitocabtagene autoleucel is currently under evaluation in the Phase III iMMagine-3 clinical trial.
The anticipated launch of these emerging CAR T-cell therapies for multiple myeloma are poised to transform the CAR T-cell therapy for multiple myeloma market landscape in the coming years. As these cutting-edge CAR T-cell therapies for multiple myeloma continue to mature and gain regulatory approval, they are expected to reshape the CAR T-cell therapy for multiple myeloma market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for multiple myeloma, visit @ CAR T-Cell Therapy for Multiple Myeloma
Recent Developments in the CAR T-Cell Therapy for Multiple Myeloma Market
- In June 2025, Arcellx announced new positive results from its pivotal Phase II iMMagine-1 trial evaluating anitocabtagene autoleucel (anito-cel) in patients with relapsed or refractory multiple myeloma (RRMM). The findings were presented in an oral session at the EHA2025 Congress in Milan on June 14, 2025.
- In June 2025, Bristol-Myers Squibb presented data from its oncology portfolio and pipeline, including a Phase III trial of Arlocabtagene autoleucel, at the 2025 American Society of Clinical Oncology (ASCO) meeting.
CAR T-Cell Therapy for Multiple Myeloma Overview
Multiple myeloma is a rare blood cancer that primarily affects plasma cells, a type of white blood cell found in the bone marrow. Under normal conditions, plasma cells produce antibodies to help fight infections. In multiple myeloma, however, these cells grow uncontrollably, displacing healthy cells and producing excessive amounts of abnormal antibodies called monoclonal proteins or paraproteins. This abnormal growth can lead to various complications, including bone pain, anemia, thrombocytopenia, leukopenia, bone damage, kidney dysfunction, and elevated calcium levels. While the precise cause of multiple myeloma remains unclear, risk factors may include age, sex, race, family history, and exposure to radiation or certain chemicals.
CAR-T cell therapy represents a next-generation immunotherapy that harnesses a patient’s own immune system to combat cancer. The treatment involves collecting T-cells from the patient, genetically engineering them to express a CAR that recognizes cancer-specific targets, expanding these modified cells, and reintroducing them into the patient. These engineered T-cells can then selectively identify and destroy cancer cells, offering a promising, potentially curative option for certain blood cancers.
CAR T-Cell Therapy for Multiple Myeloma Epidemiology Segmentation
The CAR T-cell therapy for multiple myeloma epidemiology section provides insights into the historical and current CAR T-cell therapy for multiple myeloma patient pool and forecasted trends for the leading markets. Multiple myeloma is more common in males than in females. More than 50% of males in the US are diagnosed with multiple myeloma.
Among the age-specific cases of multiple myeloma, the age group of 65 years and above has the highest number of cases, accounting for more than 70% of cases in the United States, followed by the 55–64 and 0–54 years age groups.
The CAR T-cell therapy for multiple myeloma market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Incident Cases of Multiple Myeloma
- Total Eligible Cases of Multiple Myeloma for CAR-T Cell Therapies
- Total Treatable Cases of Multiple Myeloma for CAR-T Cell Therapies
Download the report to understand CAR T-cell therapy for multiple myeloma management @ CAR T-Cell Therapy
| CAR T-Cell Therapy for Multiple Myeloma Market Report Metrics | Details |
| Study Period | 2020–2034 |
| CAR T-Cell Therapy for Multiple Myeloma Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| CAR T-Cell Therapy for Multiple Myeloma Epidemiology Segmentation | Total Incident Cases of Multiple Myeloma, Total Eligible Cases of Multiple Myeloma for CAR-T Cell Therapies, and Total Treatable Cases of Multiple Myeloma for CAR-T Cell Therapies |
| Key CAR T-Cell Therapy for Multiple Myeloma Companies | Arcellx, Kite, Juno Therapeutics, Bristol-Myers Squibb, CARsgen Therapeutics, Poseida Therapeutics, Roche, Caribou Biosciences, Gracell Biopharmaceuticals, Bluebird Bio, Johnson & Johnson, and others |
| Key CAR T-Cell Therapies for Multiple Myeloma | Anitocabtagene autoleucel, Arlocabtagene autoleucel, Zevor-cel (zevorcabtagene autoleucel), P-BCMA-ALLO1, CB-011, GC012F, ABECMA, CARVYKTI, and others |
Scope of the CAR T-Cell Therapy for Multiple Myeloma Market Report
- CAR T-Cell Therapy for Multiple Myeloma Therapeutic Assessment: CAR T-Cell Therapy for Multiple Myeloma current marketed and emerging therapies
- CAR T-Cell Therapy for Multiple Myeloma Market Dynamics: Conjoint Analysis of Emerging CAR T-Cell Therapy for Multiple Myeloma Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- CAR T-Cell Therapy for Multiple Myeloma Market Unmet Needs, KOL’s views, Analyst’s views, CAR T-Cell Therapy for Multiple Myeloma Market Access and Reimbursement
Discover more about CAR T-cell therapy for multiple myeloma in development @ CAR T-Cell Therapy for Multiple Myeloma Clinical Trials
Table of Contents
| 1 | CAR T-Cell Therapy for Multiple Myeloma Key Insights |
| 2 | CAR T-Cell Therapy for Multiple Myeloma Market Report Introduction |
| 3 | Executive Summary of CAR-T cell therapy for multiple myeloma |
| 4 | Key Events |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | CAR-T cell therapy for multiple myeloma: Market Overview at a Glance |
| 6.1 | Market Share (%) Distribution of CAR T-Cell Therapy for Multiple Myeloma in 2024 |
| 6.2 | Market Share (%) Distribution of CAR T-Cell Therapy for Multiple Myeloma in 2034 |
| 7 | CAR T-Cell Therapy for Multiple Myeloma: Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Treatment |
| 8 | Epidemiology and Patient Population of CAR-T cell therapy for multiple myeloma |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: The 7MM |
| 8.3 | Epidemiology Scenario: 7MM |
| 8.3.1 | Total Incident Cases of Multiple Myeloma in the 7MM |
| 8.3.2 | Total Eligible Cases of Multiple Myeloma for CAR-T cell Therapies in the 7MM |
| 8.3.3 | Total Treatable Cases of Multiple Myeloma for CAR-T cell Therapies in the 7MM |
| 8.4 | The US |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Marketed Therapies for CAR T-Cell Therapy for Multiple Myeloma |
| 9.1 | Key Competitors |
| 9.2 | ABECMA: Bristol Myers Squibb/Bluebird Bio |
| 9.2.1 | Product Description |
| 9.2.2 | Regulatory Milestones |
| 9.2.3 | Other Developmental Activities |
| 9.2.4 | Clinical Development |
| 9.2.4.1 | Clinical Trials Information |
| 9.2.5 | Safety and efficacy |
| 9.2.6 | Analyst Views |
| 9.3 | CARVYKTI: Johnson & Johnson |
| List to be continued in the report… | |
| 10 | Emerging Therapies for CAR T-Cell Therapy for Multiple Myeloma |
| 10.1 | Key Competitors |
| 10.2 | Anitocabtagene autoleucel (anito-cel): Arcellx and Kite (a Gilead company) |
| 10.2.1 | Product Description |
| 10.2.2 | Other Developmental Activities |
| 10.2.3 | Clinical Development |
| 10.2.3.1 | Clinical Trials Information |
| 10.2.4 | Safety and Efficacy |
| 10.2.5 | Analyst Views |
| 10.3 | Zevor-cel (CT053, zevorcabtagene autoleucel): CARsgen Therapeutics |
| List to be continued in the report… | |
| 11 | CAR T-Cell Therapy for Multiple Myeloma Market: Seven Major Market Analysis |
| 11.1 | Key Findings |
| 11.2 | Key CAR T-Cell Therapy for Multiple Myeloma Market Forecast Assumptions |
| 11.3 | CAR T-Cell Therapy for Multiple Myeloma Market Outlook |
| 11.4 | Conjoint Analysis |
| 11.5 | Total Market Size of CAR-T cell therapy for multiple myeloma in the 7MM |
| 11.6 | Total Market Size by Therapies in the 7MM |
| 11.7 | The US CAR T-Cell Therapy for Multiple Myeloma Market Size |
| 11.7.1 | Total Market Size of CAR-T cell therapy for multiple myeloma in the US |
| 11.7.2 | The Market Size by Therapies in the US |
| 11.8 | EU4 and the UK CAR T-Cell Therapy for Multiple Myeloma Market Size |
| 11.8 | Japan CAR T-Cell Therapy for Multiple Myeloma Market Size |
| 12 | Unmet Needs of Multiple Myeloma |
| 13 | SWOT Analysis of CAR T-Cell Therapy for Multiple Myeloma |
| 14 | KOL Views of CAR T-Cell Therapy for Multiple Myeloma |
| 15 | Market Access and Reimbursement of CAR T-Cell Therapy for Multiple Myeloma |
| 16 | Bibliography |
| 17 | CAR T-Cell Therapy for Multiple Myeloma Market Report Methodology |
Related Reports
CAR T-Cell Therapy for Multiple Myeloma Clinical Trial Analysis
CAR T-Cell Therapy for Multiple Myeloma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key CAR T-cell therapy for multiple myeloma companies, including Arcellx, Kite, Juno Therapeutics, Bristol-Myers Squibb, CARsgen Therapeutics, Poseida Therapeutics, Roche, Caribou Biosciences, Gracell Biopharmaceuticals, Bluebird Bio, Johnson & Johnson, among others.
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies including Sanofi, Karyopharm Therapeutics, AbbVie, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, RAPA Therapeutics, Pfizer, Array Biopharma, Cellectar Biosciences, BioLineRx, Celgene, Aduro Biotech, ExCellThera, Janssen Pharmaceutical, Precision BioSciences, Takeda, Glenmark (Ichnos Sciences SA), Poseida Therapeutics, Molecular Partners AG, Chipscreen Biosciences, AbbVie, Genentech (Roche), Janssen Biotech, Nanjing Legend Biotech, Merck Sharp & Dohme Corp., among others.
Multiple Myeloma Clinical Trial Analysis
Multiple Myeloma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key multiple myeloma companies, including CASI Pharmaceuticals, Carsgen Therapeutics, Cartesian Therapeutics, Gracell Biotechnology Shanghai Co., Ltd., Sorrento Therapeutics, TeneoOne, Karyopharma Therapeutics, Arcellx, Poseida Therapeutics, Ichnos Sciences, Nerviano Medical Sciences, Bristol Myers Squib, Ascentage Pharma, Ionis Pharmaceuticals, Chongqing Precision Biotech Co., Ltd., CRISPR Therapeutics, AstraZeneca, IGM Biosciences, Novartis, GlaxoSmithKline, Innovent Biologics, Keymed Biociences, Starton Therapeutics, Takeda, Fate Therapeutics, Gilead Sciences, Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., Janssen Pharmaceutical, Nanjing IASO Biotechnology Co., Ltd., GPCR Therapeutics, Chimerix, among others.
Relapsing Refractory Multiple Myeloma Market
Relapsing Refractory Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key RRMM companies including AbbVie, Genentech, Amgen, Onyx Therapeutics Inc., Bristol-Myers Squibb, MedImmune LLC, Novartis Pharmaceuticals, Incyte Corporation, Takeda, among others.
Relapsing Refractory Multiple Myeloma Clinical Trial Analysis
Relapsing Refractory Multiple Myeloma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key RRMM companies, including Bristol-Myers Squibb, I-MAB Biopharma, Pfizer, Arcellx, Gilead Sciences, Novartis, Array Biopharma, Hrain Biotechnology Co., Ltd., Cartesian Therapeutics, Xencor, Takeda, Sorrento Therapeutics, Heidelberg Pharma AG, Ichnos Sciences, Allogene Therapeutics, Harpoon Therapeutics, Cellectis, Poseida Therapeutics, Regeneron Pharmaceuticals, ONK Therapeutics, TeneoOne, iTeos Therapeutics, Oricell Therapeutics, Anaveon AG, Luminary Therapeutics, Seagen Inc., Trillium Therapeutics Inc., Virtuoso BINco, Inc., Seagen Inc., Trillium Therapeutics Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
